Microbiologists at Oregon State University have developed a new technology to detect illness-causing bacteria – an advance that could revolutionize the food industry, improving the actual protection to consumers while avoiding the costly waste and massive recalls of products that are suspected of bacterial contamination but are perfectly safe.
The new approach - made possible by fundamental research on the color changes in pigment-bearing cells from Siamese fighting fish - should be easier to use, faster and more directly related to toxicity assessment than conventional approaches now used to test food for bacterial contamination and safety.
"Rapid methods are not readily available to directly assess the toxicity of bacterial contamination in a user-friendly fashion," said Janine Trempy, professor of microbiology and associate dean of the OSU College of Science. "When this new technology is commercially available, we should be able to provide a higher level of assurance to the consumer while avoiding the waste of millions of dollars worth of food that is suspected of bacterial contamination, but actually is safe."
Bacterial illnesses associated with food and water can produce symptoms ranging from mild stomach upset to severe illnesses and even death, and they are common. It's been estimated there are about 76 million illnesses of this type every year that cost the U.S. more than $10 billion.
Part of the problem is that conventional food safety testing done with DNA-based tests or antibody-based methods only indicate the presence of specific bacteria, which does not necessarily describe toxicity and the potential to cause harm. Sometimes bacteria only exhibit the behavior that can cause illness under specific environmental conditions, and it's that toxic behavior that we need to detect, Trempy said.
"Bacteria are common on exposed surfaces, including the food products we consume," Trempy said. "Simply knowing they are there doesn't completely tell you, in a direct measurement, about their potential to make you sick or whether the food is safe to eat."
Existing tests only work to detect bacteria that have already been characterized, based on a specific sequence of DNA or type of protein they produce. Such tests can't tell whether the contaminating bacteria are alive or dead, they can't directly assess their toxic potential and sometimes don't detect newly emerging or genetically rearranged strains as bacteria mutate.
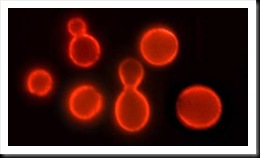
The new approach, by contrast, is built on the unusual characteristics of certain "chromatophore" or pigment bearing cells, called erythrophores, from Siamese fighting fish, whose response to specific toxic chemicals have been studied in detail by Trempy's collaborator, OSU biochemist Phil McFadden.
Siamese fighting fish (betta slendens). When Siamese fighting fish encounter certain stressful or threatening environmental conditions, such as exposure to toxic chemicals like mercury, the erythrophores change appearance, and the pigment moves in a characteristic pattern to an internal part of the cell. The change in pigment location in response to a toxic chemical is rapid, obvious and can be numerically described.
This research found that when Siamese fighting fish encounter certain stressful or threatening environmental conditions, such as exposure to toxic chemicals like mercury, the erythrophores change appearance, and the pigment moves in a characteristic pattern to an internal part of the cell. The change in pigment location in response to a toxic chemical is rapid, obvious and can be numerically described.
Another kind of stressful or threatening situation which also causes the location of pigment to change is the toxic threat posed by illness-causing bacteria. Some of these bacteria are associated with food.
"We discovered that the pigment bearing cells, erythrophores, respond immediately to certain food associated, toxin producing bacteria responsible for making humans sick," Trempy said. "There is potential to directly assess the toxic behavior of the contaminating bacteria, not just the simple presence of the DNA or protein of these bacteria. And this response can be easily seen under a low-power microscope and quickly quantified, numerically, to describe the intensity of the situation."
This technology can detect such important food-associated bacteria as Salmonella and Clostridium perfringens, responsible for diarrheal illnesses; Bacillus cereus, responsible for gastrointestinal illness characterized by vomiting and diarrhea, and often referred to as stomach flu, and Clostridium botulinum, which causes toxin-induced botulism, characterized by paralysis.
Further studies are needed to define the pigment bearing cell response to other important bacteria of concern, such as E. coli O157:H7 and Listeria, Trempy said. Research is also needed to immortalize a pigment bearing cell line for mass production and commercial use. These advances should be possible and progress is being made on both issues in continuing research, she said.
It's possible, Trempy said, that portable kits could be developed that would not require specialized training to use. Results would be available in minutes, convenient and would allow food processors, distributors, handlers, or even consumers to quickly assess food for contaminating bacterial toxicity.
Source:ScienceDaily